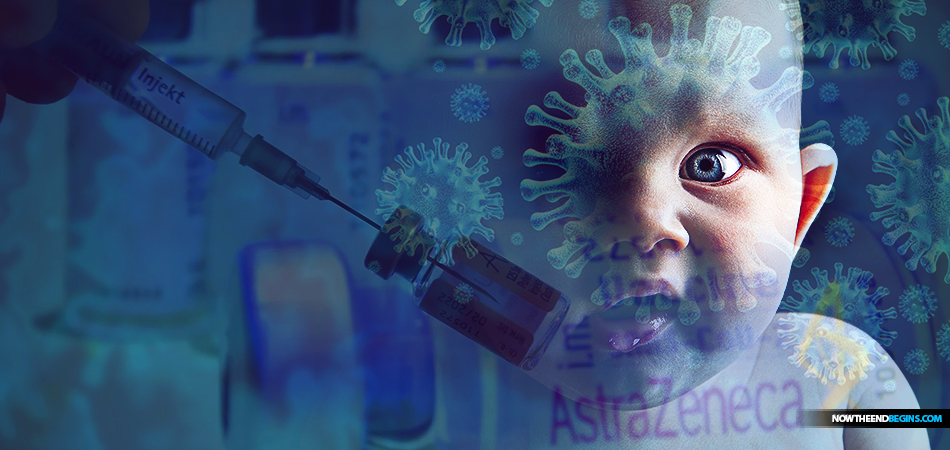
AstraZeneca which produces the Oxford COVID-19 vaccine, says it is expanding trials to children as young as six, and The Mail on Sunday can reveal that fellow COVID-19 vaccine maker Janssen, part of Johnson and Johnson, is now looking into testing on newborn babies and even pregnant women.
When we warned you all last year that the then-coming COVID-19 vaccines would be global, multiple shots for everyone on Planet Earth, many wrote to say we were deluded and paranoid conspiracy theorists, writing sensational headlines as clickbait. Six weeks into 2021 and what do we see? COVID-19 vaccines, that have not been approved outside of emergency use only, for pregnant women and newborn babies. We won't apologize for writing sensational headlines and we won't apologize for telling you the truth.
"Behold ye among the heathen, and regard, and wonder marvellously: for I will work a work in your days, which ye will not believe, though it be told you." Habakkuk 1:5 (KJB)
At this point we know that not only are they going to jab the entire world, including babies, we know that there will be multiple series of vaccinations, and we now also warn you to prepare for mandatory vaccines at regular intervals for years to come. That's where it is, that's where it's going, we officially arrived in the prophesied brave, new world. How you liking it so far? Cheer up, it's gets worse. The COVID-19 vaccine is the trojan horse, and to see what's inside the horse, go read Revelation. Not so hard to understand, but pretty hard to believe.
 ID2020 LAUNCHING THE GOOD HEALTH PASS COLLABORATIVE TO PRODUCE DIGITAL IDENTIFICATIONS FOR PEOPLE TO PROVE THEY HAVE RECEIVED THE COVID-19 VACCINE
ID2020 LAUNCHING THE GOOD HEALTH PASS COLLABORATIVE TO PRODUCE DIGITAL IDENTIFICATIONS FOR PEOPLE TO PROVE THEY HAVE RECEIVED THE COVID-19 VACCINE
AstraZeneca and Janssen said it is looking into testing COVID-19 vaccine on newborn babies
FROM THE DAILY MAIL UK: Covid-19 vaccines are set to be tested on children and potentially even newborns. Janssen last month revealed trials in adults showed its single-dose Covid vaccine is 66 per cent effective in preventing coronavirus.
The UK has ordered 30 million doses of the Janssen jabs and deliveries are expected to arrive in the second half of this year if the vaccine is approved by regulators.
It will initially extend trials to teenagers. If the jab is effective in them, it will gradually test it on younger and younger children. Hanneke Schuitemaker, who is head of viral vaccine discovery at Janssen, said discussions had begun to approve studies into the vaccine’s effects on 16 and 17-year-olds and after that ‘we will go further down to 12-year-olds but even to newborns at a certain point if all goes well’. The trials are likely to take place in the US and Canada.
AstraZeneca said a trial will begin this month on 300 volunteers aged six to 17 in Oxford, London, Southampton and Bristol.
Oxford vaccine chief Professor Andrew Pollard said: ‘It is important to establish the safety and immune response to the vaccine in children and young people as some children may benefit.’ A licensed jab for children could be available by the end of the year. READ MORE
No comments:
Post a Comment